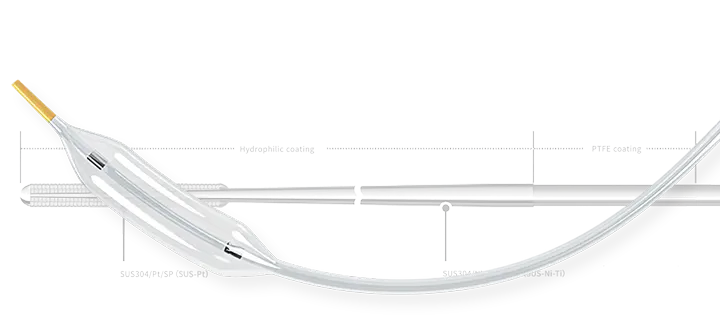
What are Asahi Intecc’s strengths?
Story - STEP3
Q.What is the source of Asahi Intecc’s competitiveness?
A.Our comprehensive production system that starts from the materials—one that is unmatched by our competitors—together with our hands-on approach, deliver speed and ensure that our prototypes reliably respond to doctors’ input.
The strength of Asahi Intecc lies in our comprehensive production system, which covers everything from the materials to the finished products. It all began in 1976. At the time, Asahi Intecc was a small town factory that manufactured and sold ultra-fine stainless steel rope used for industrial equipment in Osaka.
Later on, the company entered the medical device business, a field where it could exploit its technologies. An offer from a certain doctor turned out to be a major turning point that sparked great progress.
At the time, most treatments forCTO(chronic total occlusion) depended on surgery. It was then that a doctor who was a leading expert in catheterization asked the company if it could make guidewires that could be used forCTOtreatment. The doctor had first asked a leading overseas medical device manufacturer, but the task was one that difficult to accomplish with their level of technology at the time. Even among doctors, it was not considered common sense to treatCTOwith catheters.CTOcatheterization was particularly difficult, and success would require sophisticated skill on the part of the doctor and a new level of high-functioning guidewire that could accurately express the doctor’s fine touch.
Asahi Intecc’s sophisticated torque technology
Conventional stainless-steel wires cannot be steered with precision along circuitous paths like blood vessels (top).
Asahi Intecc torque technology achieves smooth steerability that responds with precision to the slightest movement of the physician’s fingertips (bottom).
This was an excursion into uncharted territory. However, Asahi Intecc’sfour core technologies, particularly its sophisticated torque technology, would be indispensable in faithfully conveying the feel of the instrument to the doctor’s fingertips.
We would also make the most of the ultra-fine stainless steel rope technology that we had cultivated for years. Another success factor was how quickly and precisely our prototypes — developed in a rigorous process of repeated trial and error — could respond to the input we obtained from doctors. We went back to the clinical setting again and again, seriously listening to what they said. We combined our technologies, this commitment to listening, our spirit of craftsmanship, and our determination to create a product that could treat this intractable disease, ultimately succeeding in 1995 in introducing Japan’s firstPCIguidewire forCTOtreatment. Since then, Asahi Intecc products and technologies have become well known throughout the world, thanks to the successful case results reported by doctors at academic societies, along with their overseas activities.
Unlike other medical device manufacturers that outsource parts and assembly, Asahi Intecc’s comprehensive production system spans from materials to finished products. In addition, our tradition of taking a hands-on approach has remained unchanged since the beginning. These two factors allow us to deliver speed and responsive prototyping unmatched our competitors.
Q.What supports Asahi Intecc’s competitiveness?
A.We have built an R&D and production system that ensures a global-scale competitive advantage.

ASAHI INTECC THAILAND CO., LTD.

ASAHI INTECC HANOI CO.,LTD.

TOYOFLEX CEBU CORPORATION
On the eve of Asahi Intecc’s entry into the medical device field, we found ourselves in the difficult position of needing to cut costs significantly for stainless steel rope for industrial machinery and other industrial products due to the strong yen. In response to this situation, we also moved our production base overseas (to Thailand). At the same time, in order to survive, a new kind of high value-added manufacturing was needed. With a sense of stagnation at the time, we decided to take on the challenge of launching a business in the medical device field, believing that effort would meet these requirements.
Asahi Intecc now has production bases in Thailand, Vietnam, and the Philippines. Having the Japanese sites specialize in R&D and prototyping, with the overseas plants serving as mass production sites, strengthens cost competitiveness and lets us make outsized investments in R&D.
In the ever-evolving medical industry, Asahi Intecc stays on the leading edge of technology thanks to its commitment to continuously strengthening R&D. Always looking to the future, we aspire to create more outstanding products for years to come.
-
Creating added value
-
Making the most of our strengths — four core technologies
-
Promising growth potential
-
Delivering more social contribution
-
Not competing with existing customers
Q.Can Japan’s medical devices compete worldwide?
A.As a standard bearer for “From Japan to the World” in the medical industry, we believe that our reliable technology and firm convictions can change the world’s medical care.
Doctors'comments
The introduction of revolutionaryPCIguidewires that makeCTOtreatment possible was met with much surprise by medical professionals around the world. As the success rate ofCTOtreatment increased, as shown by the cases reported at academic societies, Asahi Intecc became the center of attention.
In 1996, a request from a doctor who was a world authority in this field to sell our products in the U.S. sparked the rapid acceleration of our overseas expansion.
Even today, lively debates and heated exchanges with doctors are taking place at various medical sites. We make the most of these discussions to develop new products. Underpinning this is our totally hands-on approach. We would not be able to grasp the needs of doctors or come up with excellent products if we did not truly understand the medical clinical setting.
Thanks to these doctors, treatment results are being presented at academic societies, andefforts to disseminate our new medical technologiesare being made around the world, thereby raising the visibility of the Asahi brand.
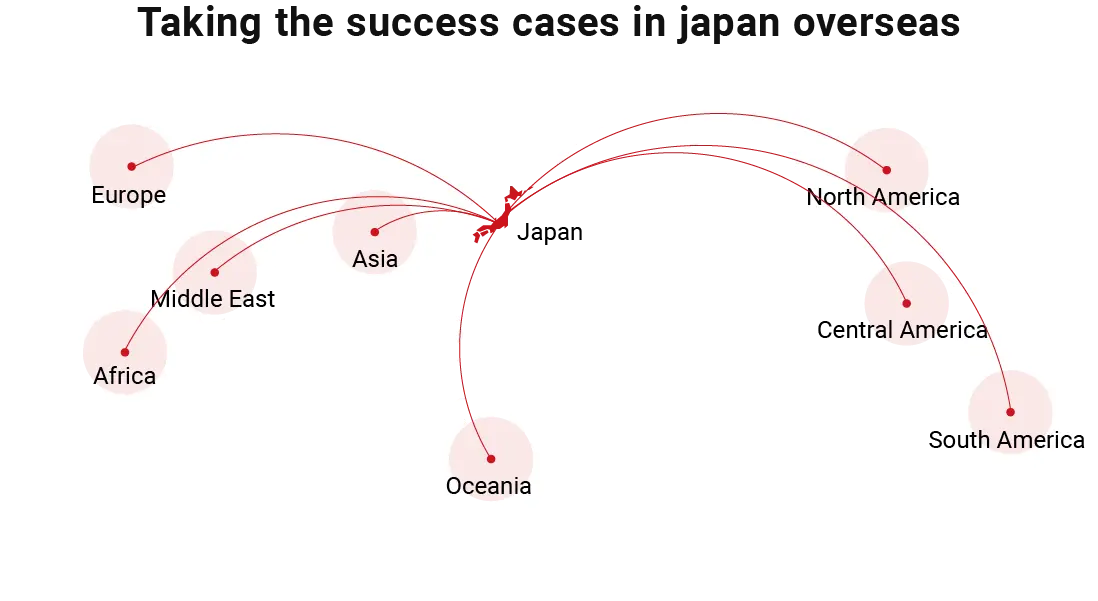
Asahi Intecc’s current leading products are for the cardiovascular system field, but treatment areas are expanding to encompass non-cardiovascular areas such as the peripheral vascular system, neurovascular system, abdominal vascular system, and digestive system.
Some of these products have already captured large shares of the market in Japan, and each year more and more of them claim a growing global reputation. We will continue to take our new technology and product success stories in Japan and actively develop them overseas.
Until now, Europe and the U.S. have taken the lead in medical industry trends. Even in Japan, which is considered to be an advanced medical nation, the majority of medical devices and pharmaceuticals used at medical sites were developed in the West.
Amid these trends, Asahi Intecc took the world by storm with the guidewires we developed in Japan. We created a new market forCTOtreatment by popularizing our product, and we look to further expand and push ahead.
WHAT IS ASAHI INTECC?
- STEP1What kind of company is Asahi Intecc?
- STEP2What is catheter treatment?
- STEP3What are Asahi Intecc’s strengths?
- STEP4What is Asahi Intecc’s vision for the future?
Find a list of indispensable terms and key words for discussing Asahi Intecc.