News Releases
News Release
Notice Regarding a definitive distribution agreement for the Exclusive Sales of Penumbra's Peripheral Vascular Thrombus Aspiration Device in the Japanese Market
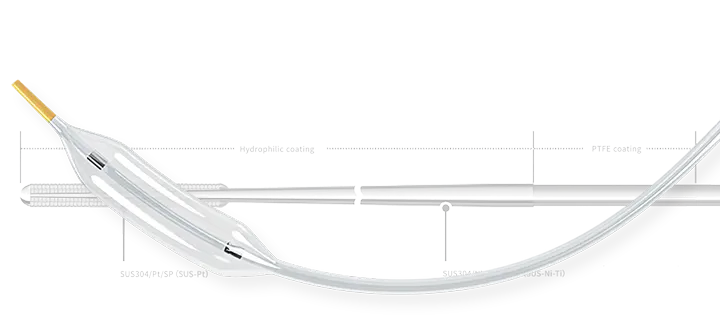
As previously announced in the "Notice Regarding a Basic Agreement for the Exclusive Sales of Penumbra's Peripheral Vascular Thrombus Aspiration Device in the Japanese Market" dated September 22, 2022, Asahi Intecc Group and Penumbra Inc. (hereinafter referred to as “Penumbra”) have signed a basic agreement for the exclusive sales of peripheral vascular thrombus aspiration device "Indigo™ and Lightning™ system" in the Japanese market by its consolidated subsidiary, Asahi Intecc J-sales, Inc. Penumbra and Asahi Intecc Group have been in discussions since then, and today, on November 2, a definitive distribution agreement has been signed.
Furthermore, Peripheral vascular thrombus aspiration device "Indigo™ system" manufactured by Penumbra, has been approved in April 2023 under the Japanese Pharmaceutical and Medical Device Act. Apart from this matter, there are no significant changes in the background or content of the basic agreement as previously announced in the press release.
Furthermore, Peripheral vascular thrombus aspiration device "Indigo™ system" manufactured by Penumbra, has been approved in April 2023 under the Japanese Pharmaceutical and Medical Device Act. Apart from this matter, there are no significant changes in the background or content of the basic agreement as previously announced in the press release.