News Releases
News Release
Asahi Intecc Receives CTO Indication from the U.S. FDA for Selected Products
Asahi Intecc was notified on February 12, 2018 that the FDA has approved a CTO (Chronic Total Occlusion) indication for selected products. Asahi Intecc performed a clinical study of 163 patients at 12 centers, which ran from January 2015 to January 2016, to prove the safety and efficacy of our products in CTO cases. The data from this study was submitted to the FDA to support the application to include an indication for CTO cases on the studied devices.
The following devices have received the CTO indication:
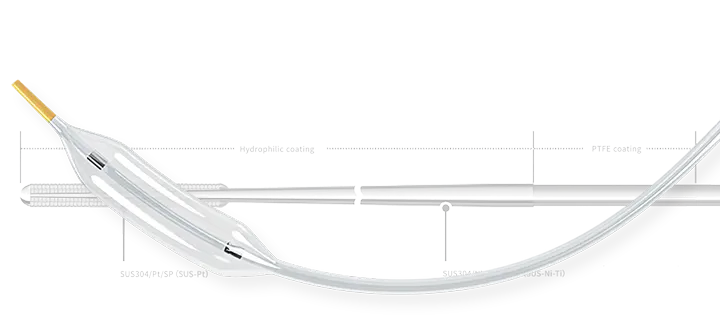
PTCA Guide Wires: ASAHI Gaia series, MIRACLEBROS/ASAHI Miracle series, CONFIANZA/Conquest series, FIELDER XT, FIELDER XT-A, FIELDER XT-R, ULTIMATEbros 3.
Microcatheters: ASAHI Corsair, Corsair Pro microcatheters
The CTO indication will give physicians greater freedom to select Asahi devices for CTO cases, and allows Asahi Intecc to promote these devices for use in CTO cases. We look forward to providing physicians with the best tools to treat these complex disease states.
The following devices have received the CTO indication:
PTCA Guide Wires: ASAHI Gaia series, MIRACLEBROS/ASAHI Miracle series, CONFIANZA/Conquest series, FIELDER XT, FIELDER XT-A, FIELDER XT-R, ULTIMATEbros 3.
Microcatheters: ASAHI Corsair, Corsair Pro microcatheters
The CTO indication will give physicians greater freedom to select Asahi devices for CTO cases, and allows Asahi Intecc to promote these devices for use in CTO cases. We look forward to providing physicians with the best tools to treat these complex disease states.